The ideal gas law specifies that the volume occupied by a gas depends upon the amount of substance as well as temperature and pressure. Standard temperature and pressure -- usually abbreviated by the acronym STP -- are 0 degrees Celsius and 1 atmosphere of pressure. Parameters of gases important for many calculations in chemistry and physics are usually calculated at STP. An example would be to calculate the volume that 56 g of nitrogen gas occupies. Eventually, these individual laws were combined into a single equation—the ideal gas law—that relates gas quantities for gases and is quite accurate for low pressures and moderate temperatures.
We will consider the key developments in individual relationships , then put them together in the ideal gas law. The moles to liters/liters to moles conversion is straightforward and is based on the fact that the ideal gas equation is a good approximation for many common gases at standard temperature and pressure. Thus, we can rearrange terms in the ideal gas equation and write them like this . The behavior of gases can be described by several laws based on experimental observations of their properties.
The pressure of a given amount of gas is directly proportional to its absolute temperature, provided that the volume does not change (Amontons's law). The volume of a given gas sample is directly proportional to its absolute temperature at constant pressure (Charles's law). The volume of a given amount of gas is inversely proportional to its pressure when temperature is held constant (Boyle's law). Under the same conditions of temperature and pressure, equal volumes of all gases contain the same number of molecules (Avogadro's law).
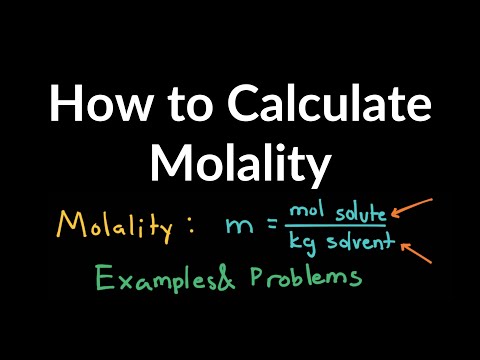
Molality is an intensive property of solutions, and it is calculated as the moles of a solute divided by the kilograms of the solvent. Unlike molarity, which depends on the volume of the solution, molality depends only on the mass of the solvent. Since volume is subject to variation due to temperature and pressure, molarity also varies by temperature and pressure. In some cases, using weight is an advantage because mass does not vary with ambient conditions.
For example, molality is used when working with a range of temperatures. It is important to note that the molarity is defined as moles of solute per liter of solution, not moles of solute per liter of solvent. This is because when you add a substance, perhaps a salt, to some volume of water, the volume of the resulting solution will be different than the original volume in some unpredictable way. To get around this problem chemists commonly make up their solutions in volumetric flasks. These are flasks that have a long neck with an etched line indicating the volume. The solute is added to the flask first and then water is added until the solution reaches the mark.
The flasks have very good calibration so volumes are commonly known to at least four significant figures. To calculate the molarity of a solution, the number of moles of solute must be divided by the total liters of solution produced. If the amount of solute is given in grams, we must first calculate the number of moles of solute using the solute's molar mass, then calculate the molarity using the number of moles and total volume. To calculate molarity, divide the number of moles of solute by the volume of the solution in liters.
If you don't know the number of moles of solute but you know the mass, start by finding the molar mass of the solute, which is equal to all of the molar masses of each element in the solution added together. Once you have the molar mass, multiply the number of grams of solute by 1 over the molar mass to convert the grams into moles. Finally, divide the number of moles by the volume of the solution to get the molarity. The ideal gas law formula states that pressure multiplied by volume is equal to moles times the universal gas constant times temperature. The units of molar concentration are moles per cubic decimeter.
They are noted as mol/dm³ as well as M (pronounced "molar"). The molar concentration of solute is sometimes abbreviated by putting square brackets around the chemical formula of the solute, e.g., the concentration of hydroxide anions can be written as [OH⁻]. In many older books or articles, you can find different units of molar solutions – moles per liter (mol/l). Remember that one cubic decimeter equals to one liter, so these two notations express the same numeric values.
In chemistry and physics a mole describes an amount of a substance in grams equal to its atomic mass. For example, one mole of aluminum has a mass of 13 grams since it has an atomic mass of 13. Also, one mole of a substance contains Avogadro's number of atoms, namely 6.02 times 10 to the power 23. The molarity, or concentration of a solution, equals the number of moles in the solution divided by its volume.
Conversion between moles, molarity and volume is performed frequently in science problems. Gases whose properties of P, V, and T are accurately described by the ideal gas law are said to exhibit ideal behavior or to approximate the traits of an ideal gas. An ideal gas is a hypothetical construct that may be used along with kinetic molecular theory to effectively explain the gas laws as will be described in a later module of this chapter. Although all the calculations presented in this module assume ideal behavior, this assumption is only reasonable for gases under conditions of relatively low pressure and high temperature. In the final module of this chapter, a modified gas law will be introduced that accounts for the non-ideal behavior observed for many gases at relatively high pressures and low temperatures. An aqueous solution consists of at least two components, the solvent and the solute .
Usually one wants to keep track of the amount of the solute dissolved in the solution. One could do by keeping track of the concentration by determining the mass of each component, but it is usually easier to measure liquids by volume instead of mass. To do this measure called molarity is commonly used.
Molarity is defined as the number of moles of solute divided by the volume of the solution in liters. Both terms are used to express the concentration of a solution, but there is a significant difference between them. While molarity describes the amount of substance per unit volume of solution, molality defines the concentration as the amount of substance per unit mass of the solvent. In other words, molality is the number of moles of solute per kilogram of solvent .
The SI base unit for the quantity of substance is the mole, which is abbreviated mol. And liters are an SI unit of volume equal to one cubic decimeter. This relationship between temperature and pressure is observed for any sample of gas confined to a constant volume. An example of experimental pressure-temperature data is shown for a sample of air under these conditions in Figure 9.11. Avogadro was an Italian Physicist who first described the Avogadro constant as a hypothesis in 1811. He was trying to understand why in chemical reactions involving gases the observation that equal volumes of different gases had the same number of moles.
This was found true even when the masses were very different. The idea that a mole of any substance has exactly the same number of atoms no matter what the substance is made of was explained by Avogadro and his name has stuck to his number ever since. Where Z is the gas compressibility factor, which is a useful thermodynamic property for modifying the ideal gas law to account for behavior of real gases.
The above equation is basically a simple equation of state . At standard temperature and pressure , 1 mole of ideal gas is equal to 22.4 liters. Thus, the conversion ratio used in the formula below is 22.4. Molecules are composed of several atoms, for example a carbon dioxide molecule is made up of 1 carbon atom and 2 oxygen atoms. The molecular weight (or molecular mass or relative molecular mass ) is the sum of the atomic weights of all the atoms in the molecule. Note that the molar heat capacity of an ideal gas mixture is the mole fraction weighted average of the pure component values, i.e.
One mole of any gas occupies the same volume when measured under the same conditions of temperature and pressure. In this experiment, the volume of one mole of hydrogen is calculated at room temperature and pressure. The volume and temperature are linearly related for 1 mole of methane gas at a constant pressure of 1 atm. If the temperature is in kelvin, volume and temperature are directly proportional.
Charles's law states that the volume of a given amount of gas is directly proportional to its temperature on the kelvin scale when the pressure is held constant. Where x i is the mole fraction of the ith component, M i is the molecular mass of the ith component and ρmixture is the mixture density at the given temperature and pressure. Unlike converting the mass in grams to moles, converting the volume of gas in liters to moles uses a simple conversion formula. One major benefit of the behavior of gases is that the volume of one ideal gas in a mixture of ideal gases is equivalent to its mole fraction. For all practical purposes, the volume fractions and the mole fractions of the components of an ideal gas mixture are interchangeable.
You can get your moles by taking the molar mass of each of the elements in the solute and adding them together. Do it; the answer is in moles because the grams cancelled out.Then, go ahead and do your formula. This equation is read as 2 moles of sodium hydrogencarbonate decomposes to give 1 mole of sodium carbonate, 1 mole of water and 1 mole of carbon dioxide gas.
This means equal amounts of moles of gases occupy the same volume under the same conditions of temperature and pressure. So you are not confused with similar chemical terms, keep in mind that molarity means exactly the same as molar concentration . Molarity expresses the concentration of a solution. It is defined as the number of moles of a substance or solute, dissolved per liter of solution (not per liter of solvent!).
MolarityThe concentration of a substance in solution, expressed as the number moles of solute per liter of solution. The interest stems from that accurate measurements of the unit cell volume, atomic weight and mass density of a pure crystalline solid provide a direct determination of the Avogadro constant. Two solutions that have the same molarity will have the same number of molecules of the chemical per liter but are likely to contain differing masses of that chemical per liter to achieve this.
Whereas two solutions at the same concentration will have the same mass of the chemical per liter of solution but are therefore likely to have differing numbers of molecules of that chemical per liter. Provided some additional information is known, one value can be deduced from the other using the equations below. We use the total pressure of the gas in Equation 4.18 and not the partial pressure because we are using the volume fraction based on the total volume and total pressure of our system. If we used the total volume of the system instead of the volume fraction, then we would use the partial pressure of the gas and Equation 4.18 would look something like Equation 4.21. Now that you have the molar mass of the solute, you need to multiply the number of grams of solute in the solution by a conversion factor of 1 mole over the formula weight of the solute.
This will give you the number of moles of the solute for this equation. To calculate molarity, you can start with moles and volume, mass and volume, or moles and milliliters. Plugging these variables into the basic formula for calculating molarity will give you the correct answer. The ideal gas equation contains five terms, the gas constant R and the variable properties P, V, n, and T. Specifying any four of these terms will permit use of the ideal gas law to calculate the fifth term as demonstrated in the following example exercises. This relationship shows us that if we increase the moles of gas, n, by adding more gas while maintaining the same temperature and pressure, the volume of gas, V, will also increase.
Examples and practice problems of solving equation stoichiometry questions with gases. We calculate moles with 22.4 L at STP, and use molar mass and mole ratios to figure out how many products or reactants we have. The most common molar volume is the molar volume of an ideal gas at standard temperature and pressure (273 K and 1.00 atm). Molarity is not the same as concentration, although they are very similar. Concentration is a measure of how many moles of a substance are dissolved in an amount of liquid, and can have any volume units. Molarity is a type of concentration, specifically moles per liter of solution.
Chemists use many different units for describing concentration. However, the term molarity, also known as molar concentration, is the most common way of expressing the concentration. When the reactants are expressed in mole units, it allows them to be written with integers in chemical reactions.
First, let's take a closer look at what is the mole, so we can move on later to find what is molarity. In a mixture of ideal gases, the mole fraction can be expressed as the ratio of partial pressure to total pressure of the mixture. Molarity indicates the number of moles of solute per liter of solution (moles/Liter) and is one of the most common units used to measure the concentration of a solution.
The relative formula mass of a compound is calculated by adding together the relative atomic mass values for all the atoms in its formula. Moles are units used to measure substance amount. Thus, the amount of substance in moles is equal to the volume of ideal gas in liters divided by the conversion ratio of 22.4 L/mol. Air - Molecular Weight and Composition - Dry air is a mixture of gases where the average molecular weight can be calculated by adding the weight of each component.
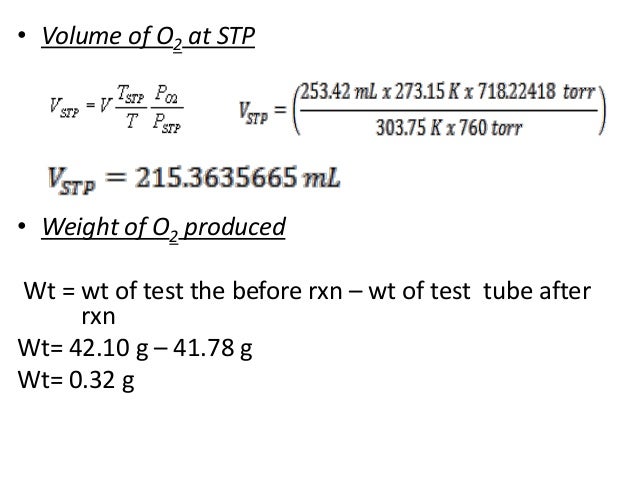
Ions with large hydrophobic surfaces such as tertaalkylammonium ions in effect increase hydrogen bonding in the adjacent water structure, which entails a further contribution, V∞caged. If this effect prevails, the ion is a structure-making ion, in contrast to the structure-breaking ions with a large V∞disorder term. Temperature is sometimes measured with a gas thermometer by observing the change in the volume of the gas as the temperature changes at constant pressure. The hydrogen in a particular hydrogen gas thermometer has a volume of 150.0 cm3 when immersed in a mixture of ice and water (0.00 °C). When immersed in boiling liquid ammonia, the volume of the hydrogen, at the same pressure, is 131.7 cm3. Find the temperature of boiling ammonia on the kelvin and Celsius scales.
One mole of any gas occupies 24 dm3 at room temperature and pressure. One mole of water has a mass of 18 grams and volume of 18 milliliters or 0.018 liters.How much is a mole of water? A mole of water is Avogadro's number of water molecules.
Avogadro's number is so large that it can be difficult to imagine its size. Finding the mass and volume of one mole of water is a great way to relate the units to something familiar. Here is the calculation for the the mass and volume of one mole of water. The volume units must be the same for both volumes in this equation. In general, M1 usually refers to as the initial molarity of the solution. V1 refers to the volume that is being transferred.
M2 refers to the final concentration of the solution and V2 is the final total volume of the solution. Likewise, the only way to decrease the volume of gas, V, while maintaining the same temperature and pressure, is to decrease the moles of gas, n, that are present, that is, remove some of the gas. Likewise, if we decrease the moles of gas, n, by removing some of the gas while maintaining the same temperature and pressure, the volume of gas, V, will also decrease. Well, if we assume that the temperature and pressure are held constant, then we can substitute our values into the equation. 10 L is our initial volume and 20 L is our final volume.






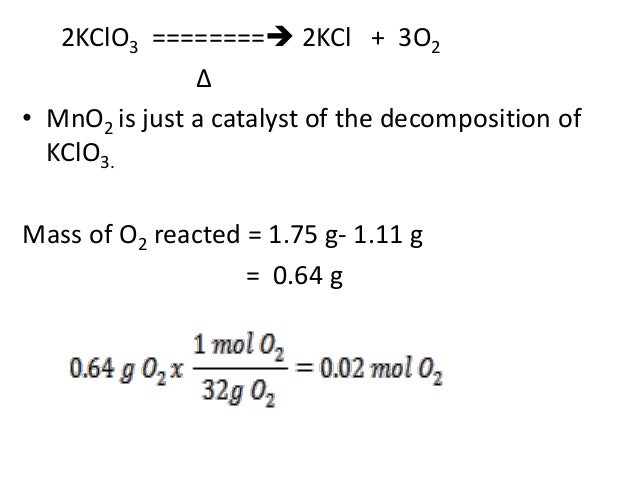
















No comments:
Post a Comment
Note: Only a member of this blog may post a comment.